 Chrissy Teigen revealed she's suffered a miscarriage after being hospitalised for "excessive bleeding."
Chrissy Teigen revealed she's suffered a miscarriage after being hospitalised for "excessive bleeding."from Top Lifestyle News- News18.com https://ift.tt/34hOG9P
 Chrissy Teigen revealed she's suffered a miscarriage after being hospitalised for "excessive bleeding."
Chrissy Teigen revealed she's suffered a miscarriage after being hospitalised for "excessive bleeding."Google has launched a series of products including the Chromecast with Google TV, Pixel 5, Pixel 4a 5G and a Nest smart speaker at a recent n event. The two smartphones feature a dual rear camera setup, punch hole display that houses an 8 MP front camera. Both the smartphones are powered by Snapdragon 765G chipset. As for Chromecast with Google TV, the only thing that sets it apart from its previous generations is that it comes with a remote and its own user interface.
Google Pixel 5 is priced at $699 (approx Rs 51,400) and Pixel 4a 5G will cost you $499 (approx Rs 37,000). Both smartphones will be available in Just Black and Sorta Sage colour variants. Notably, the two handsets will not make their way to India.
The Chromecast with Google TV is priced at $49.99 (approx Rs 3,700) in the US. The company is yet to reveal if it will be launched in India or not.
The smartphone features a 6-inch full-HD+ OLED display that has a resolution of 1,080 x 2,340 pixels and a 90 Hz refresh rate. It is powered by Snapdragon 765 chipset and offers 8 GB RAM and 128 GB internal storage. It runs on Android 11 and sports a rear-mounted fingerprint scanner.
For photography, it comes with a dual rear camera setup that houses a 12 MP primary camera and a 16 MP ultra-wide-angle lens. For selfies, it comes with an 8 MP front camera.
In terms of battery, Pixel 5 is equipped with a 4,080 mAh battery that comes with support for 18W fast charging and reverse charging.
Pixel 4a 5G features a 6.2-inch full-HD+ OLED display that comes with a resolution of 1,080 x 2,340 pixels. Just like pixel 5, it is powered by Snapdragon 765 chipset and offers 6 GB RAM and
In terms of camera, just like Pixel 5, you will get a 12 MP primary sensor and a 16 MP ultra-wide-angle lens. The smartphone houses an 8 MP front camera for selfies.
As of battery, it comes with a 3,885 mAh battery that supports 18W fast charging.
The new dongle comes with a voice control remote that has two AAA batteries. The remote also comes with shortcut keys for also shortcut buttons for Google Assistant, YouTube, and Netflix. It runs on a new Google TV platform that runs on Android TV OS. The dongle has a Type-C power adapter and supports 4K HDR, Dolby Atmos, Dolby Digital Plus, Dolby Vision, HDR10+ and DTSX.
The device comes in Snow, Sunrise, and Sky colour variants.
The new Google UI categorises content on the basis of genres, suggestions based on your history. As per a report by GSMArena, in terms of layout, it looks a lot like Apple TV or Amazon Fire TV UI. There is a Watchlist feature in Google UI that lets users add and remove content to watch list from any app or any device.
Vivo has teased the launch of the Vivo V20 series in India. On Twitter, Vivo India posted a short teaser video teasing the new series of smartphone. The video teaser suggests that the V20 series smartphones will be "ultra-slim". The caption of the teaser reads: “Tick, tock. Tick, tock. Time’s up for bulky smartphones! Are you ready for an ultra-slim delight?” Additionally, Vivo also changed the cover photo on its Twitter profile page, which suggests that the smartphone will come in three colour variants – black, white, and blue-purple gradient.
Tick, tock. Tick, tock.🕛
Time's up for bulky smartphones! Are you ready for an ultra-slim delight? #ComingSoon #DelightEveryMoment #vivoV20Series pic.twitter.com/Fb2rMTHnXo— Vivo India (@Vivo_India) September 28, 2020
Meanwhile, on its official website, Vivo has reealed the complete specifications of the V20 series.
As per Vivo, the V20 series will include three smartphones: the Vivo V20 Pro, Vivo V20, and Vivo V20 SE.
The Vivo V20 SE and the Vivo V20 Pro have already been launched earlier this month in Malaysia and Thailand, respectively. We already know that the smartphones will be 7.38 mm thick – which is one of the highlights of the series.
Additionally, the website confirms that the Vivo V20 will be powered by Qualcomm Snapdragon 720G processor and support 33 W FlashCharge. The smartphone will sport a 44 MP eye autofocus, super night mode and a 64 MP rear camera. The front camera will support 4K selfie video eye autofocus.
The Vivo V20 series will also come with Android 11 operating system, out-of-the-box.
A promotional page for the Vivo V20 has been also created on Flipkart, suggesting that the device will be exclusively available on the e-commerce platform in India. The device will be also sold via offline stores.
Since the start of the Covid-19 pandemic, scientists have churned out research papers at an unprecedented rate. Among the most closely watched studies are clinical trials, which are designed to determine whether a given treatment is safe and effective in humans. Hundreds of these trials have been approved around the world over the past six months. And yet one group of patients — pregnant and lactating mothers — are being left out. An analysis of 927 Covid-19 clinical trials across Asia, Europe, and North America published online in May found more than half explicitly excluded pregnant women. Others simply failed to mention that pregnant women could enroll.
Only 16 trials — less than 2 percent — were pregnancy-specific, meaning they aimed to evaluate a treatment’s effects on fetuses and expectant mothers. This means that even as researchers learn which treatments work for most people, there will be a gap in the medical community’s understanding of how these treatments perform during pregnancy.
The problem is not unique to studies of Covid-19. For decades, expectant mothers have been considered a vulnerable group to be shielded from potential harms of research for the sake of their fetuses’ health. This view stems, in part, from tragedies caused by two now-infamous drugs that were widely prescribed to pregnant women in the mid-20th century: thalidomide, which caused thousands of children around the world to be born with flipper-like limbs and other birth defects, and diethylstilbestrol (DES), which was linked to higher rates of cancer in both mothers and the daughters born to them.
But some experts say that regulations aiming to prevent such disasters could cause damage of a different kind. The near absence of clinical trial data leaves pregnant women “widely exposed” to drugs that have not been vetted for use in pregnancy, says bioethicist and OB/GYN Anne Lyerly of the University of North Carolina. Excluding pregnant women from clinical trials doesn’t eliminate risk, she points out. It simply shifts the risk from research studies to the doctor’s office, where pregnant women receive treatments rarely supported by robust data about how they will respond and whether the drugs are effective in pregnancy.
In fact, today, little is known about how the vast majority of drugs affect maternal and fetal health. One study, published in the journal Obstetrics and Gynecology in 2002, found that 90 percent of the drugs approved by the U.S. Food and Drug Administration from 1980 to 2000 had an “undetermined” potential to cause fetal malformations. “Inadequate information is available for pregnant women and their physicians” to decide whether the benefits exceed the risks for most drugs introduced during the study period, the authors concluded.
Over the past few years, federal agencies have made changes that, in principle, should help to include more pregnant women in clinical trials. But closing the data gap, some say, will also require a shift in how risk is conceptualized. Currently, when it comes to research, “the focus is so often on fetal risk that we’ve failed to recognize the benefits of including pregnant women,” says bioethicist and OB/GYN Amina White, also at the University of North Carolina.
Pregnant women should have access to drugs that have been vetted for safety, says White. “It’s an issue of justice.”
Today’s clinical research is guided by a set of principles laid out in 1979 by a federal commission that had been created five years before with the aim of bolstering the ethical underpinnings of research conducted on humans. Key tenets outlined in the final report included beneficence, the notion that researchers have an obligation to maximize benefits and minimize risks, and justice, which ensures that the benefits and burdens of research are equitably distributed to all populations.
Fears about whether administering drugs during pregnancy violates these principles can be traced back to problems with drugs developed decades earlier. Launched in 1938, DES was marketed as a preventive for miscarriages and premature births. The drug was widely used, despite a 1953 study finding no effect on either outcome. In 1971, researchers found the drug caused a rare vaginal cancer in girls born to those who took DES while pregnant, leading to an FDA warning against its use in pregnancy. Subsequent research revealed additional risks to pregnant women who had been prescribed the drug and their daughters.
Beginning in 1957, another drug, thalidomide, was distributed to thousands of pregnant women around the world as a remedy for morning sickness. (Notably, the FDA did not approve the drug for sale in the U.S. at the time due to lack of evidence for its safety.) In the 1960s, reports began to emerge of severe birth defects in babies born to women who had taken the drug; researchers and regulators eventually recognized it as the cause of limb malformations in babies and stopped its use in pregnancy. “Those events clearly had a bearing on recommendations that ended up being codified in the federal regulations we have today,” White says.
Thalidomide’s unusual risks were partly a result of unfortunate timing — the symptoms of morning sickness typically coincide with the time when limbs are forming in the womb. Generally, drugs taken early in pregnancy have greater odds of affecting the formation of organs and other body parts, while drugs taken later in a pregnancy may affect brain development and birth weight. “The concern that you may disrupt something in these early stages” — with potentially lifelong consequences — “carries a huge weight,” says pediatrics researcher Christina Chambers of the University of California, San Diego.
In 1977, the FDA issued guidelines that excluded pregnant women and women “with childbearing potential” from phase I and phase II clinical trials, where new drugs are tested for their safety and efficacy. Inclusion in some studies became possible with the passage of the NIH Revitalization Act of 1993, which sought to increase gender and racial diversity in clinical trials.
But while pregnant women are now able to enroll in studies, concerns about their participation linger. Federal regulations currently require any study involving pregnant women to meet 10 criteria, including that, “where scientifically appropriate,” data first be collected on pregnant animals and non-pregnant human subjects to assess risk, and that any risk to mother or fetus be “the least possible for achieving the objectives of the research.”
Whether these protections would catch another thalidomide-like drug before tragedy occurs is unclear. “I would hope it would be detected,” says OB/GYN Beatrice Chen, vice chair of the Institutional Review Board at the University of Pittsburgh. Chen notes, however, that sometimes a drug’s risk to mother and fetus isn’t discovered until after it comes to market.
This is why some researchers say regulators have taken the wrong lessons from the thalidomide tragedy. “It wasn’t that research was done and it was harmful,” says Lyerly. “The problem,” she says, is that thalidomide “was distributed for widespread use” without first testing it for safety.
Recent regulatory changes have been made to include more pregnant women in studies. One crucial shift is classifying pregnant people as “medically complex” rather than “vulnerable.” The latter is a term usually reserved for prisoners and other groups at risk of exploitation or unable to make decisions for themselves, says bioethicist Maggie Little of Georgetown University. In 2018, the U.S. Department of Health and Human Services removed pregnant women from its list of subjects “vulnerable to coercion or undue influence.” Draft guidance from the FDA published in 2018 avoids the term entirely in recognition of the need to include pregnant women in clinical research.
“The change that’s now needed is a cultural shift,” Little says. “Instead of thinking it’s unethical to do research with pregnant women,” researchers should consider that “it’s unethical not to include them.”
But federal regulations don’t mandate inclusion. The final call rests with the specialists on institutional review boards, who still tend to err on the side of caution, says University of Pennsylvania OB/GYN Michal Elovitz. For example, when Elovitz and her colleagues launched a trial for convalescent plasma to treat Covid-19, they were asked to submit extensive support for their decision to include pregnant and lactating mothers. Plasma transfusions are commonly used to help with pregnancy-related problems, such as certain immune disorders or bleeding, so the reams of evidence they had to provide to allow pregnant women to participate felt “a bit excessive,” Elovitz says. In such instances, regulators need to reconsider what evidence of safety they consider sufficient for a trial to be conducted, she adds. “We have to be careful about where benevolence crosses over to patriarchy.”
Trials that include pregnant women are often costlier, and they take longer to launch, given the additional safety and monitoring requirements. To glean evidence of whether a drug is safe and effective in pregnancy, researchers also need to enroll sufficient numbers of pregnant mothers, which can increase the size of a trial. In addition, drug makers worry about insurance for liability if harm occurs, Lyerly says.
With little incentive for inclusion, most drugs on the market today are approved without any data on their use in human pregnancy. As a result, that data is still usually obtained after the drugs reach market, where women’s experiences and possible side effects are tracked over time in registries. But this design creates biases, experts say, since women are only likely to report severe reactions that they perceive as being related to their use of a drug while pregnant. Milder reactions such as headaches or fatigue may go unnoticed and registries rarely, if ever, keep score of instances where neither mother nor offspring experienced negative side effects. “That limits the generalizability of any ‘evidence’ that appears to come from registries,” White says.
There is growing acknowledgement that drugs are not the only risk to fetuses — maternal disease is too. If left untreated, for example, diabetes increases the risk of congenital abnormalities from 3 percent to as high as 25 percent. Untreated hypertension can cause babies to be born several weeks premature. But until recently, researchers didn’t have enough data to know which existing medications could most effectively minimize these risks and whether drugs that were being prescribed carried risks of their own.
To identify solutions, the NIH began to fund studies of how drugs were metabolized in pregnancy. Simply observing metabolic changes in blood samples yielded new information, such as finding that quicker kidney filtration during pregnancy meant that pregnant women needed higher doses of drugs that were filtered out of the blood by the kidneys. This was true of both a common diabetes drug and a new HIV drug.
Surveys suggest that many pregnant women are keen to participate in clinical research. In 2013, researchers tested a common hypertension drug to treat pre-eclampsia, a life-threatening pregnancy complication. Although already coping with high-risk pregnancies, study participants said they enrolled because they preferred assuming the risk of any potential side effects to access the drug’s benefits to delivering a baby at 34 weeks — the likely outcome of leaving their condition untreated.
In other cases, patients find themselves unable to access treatments they need outside of trials. Thirty-seven-year-old Marisa Sprowles was born with hepatitis C, the fallout of a blood transfusion her mother had after knee surgery as a child that had then been passed on to her. The viral infection is curable, although treatments have historically been expensive and only covered by insurance for patients with advanced symptoms. Newly pregnant, Sprowles jumped at the opportunity to access treatment via a small clinical trial at Magee-Womens Research Institute in Pittsburgh. Her own infection was cured, and her and her husband’s now 2-year-old son was born disease-free.
“There’s been a slight movement of the needle,” says Sylvia LaCourse, an infectious disease researcher at the University of Washington in Seattle. One large U.K.-based study of Covid-19 drugs, the Recovery trial, does include pregnant and lactating women. And in response to feedback, the World Health Organization amended the Covid-19 clinical trial it’s sponsoring to permit the inclusion of pregnant women. “Whenever there’s a successful large-scale trial that includes pregnant women, it sets a precedent,” says LaCourse. Even a small number of pregnant study participants, she adds, can illuminate whether a drug might behave differently during pregnancy.
Of course, greater inclusion doesn’t eliminate risk. But in a closely monitored study, it does make it possible that potentially harmful drugs can be caught before they affect thousands in unfathomable ways. Clinical research minimizes the potential for harm, Lyerly says. “There’s no way to take the risk away entirely. But if you don’t look, it doesn’t mean it goes away.”
Jyoti Madhusoodanan is a science writer based in Portland, Oregon.
This article was originally published on Undark. Read the original article.
 The name Mahen Vakil may not automatically ring a bell in the minds of the most ardent Bollywood buffs, but if we told you he was a vital cog in the production of such films as Darr (1993), Dilwale Dulhania Le Jayenge (1995), Dil To Pagal Hai (1997) and Mohabbatein (2000), you would surely do a double take.
The name Mahen Vakil may not automatically ring a bell in the minds of the most ardent Bollywood buffs, but if we told you he was a vital cog in the production of such films as Darr (1993), Dilwale Dulhania Le Jayenge (1995), Dil To Pagal Hai (1997) and Mohabbatein (2000), you would surely do a double take.Earlier this month, Microsoft announced that Xbox Game Pass Ultimate and PC members will be provided with an EA Play membership at no additional cost. Now, just in time for the official launch of the Xbox Series S and Series X, Microsoft has shared more details about the same. As per the company, Xbox Game Pass Ultimate subscribers will be able to access the complimentary EA Play subscription starting 10 November. The next-generation Microsoft gaming consoles will also be released the same day.
This means, that an Xbox Game Pass Ultimate subscription will soon include access to Microsoft’s library of games, an Xbox Live Gold subscription, xCloud and EA Play. The Ultimate subscription is priced at Rs 699 per month, and the PC membership is priced at Rs 489 per month. It costs $14.99 per month. Do note, if you just subscribe to the Xbox Game Pass at Rs 489 per month, you won’t get EA Play.
Additionally, Microsoft has clarified that if you’re already paying for EA Play and Xbox Game Pass Ultimate subscription, your EA Play subscription will be cancelled and your remaining time will be converted to Xbox Game Pass Ultimate. As per the FAQ, if you have between 50 days to three months left on your subscription, you’ll receive one month of Xbox Game Pass Ultimate, and if you between four to six months remaining, you’ll receive two months of Xbox Game Pass Ultimate. You can refer to the FAQs for more details.
Players who have the Ultimate membership in the Xbox Game Pass will be able to use the subscription on a variety of consoles like the Xbox One, Xbox Series X, and Xbox Series S. On the other hand, the members with Xbox Game Pass for PC now get EA Play on Windows 10 PCs. The offer will go live later this year.
The EA Play subscription will bring over 60 of EA’s popular titles such as FIFA 20, Titanfall 2 and Need for Speed Heat to Xbox Game Pass. Titles from popular EA franchises like Battlefield, Mass Effect, Skate, and The Sims will also be available.
An interdisciplinary team of researchers from the Indian Institute of Science (IISc) has used a 3D tumour model and magnetically-driven nanomotors to probe the microenvironment of cancer cells. The team consisted of researchers from the Centre for Nano Science and Engineering (CeNSE) and Department of Molecular Reproduction, Development and Genetics (MRDG), Bengaluru-based IISc said a media statement on Wednesday. In their work, published in Angewandte Chemie, the team steered helical nanomotors remotely via an external magnetic field through the tumour model to sense, map and quantify changes in the cellular environment.
The model comprises both healthy and cancer cells embedded within a reconstituted basement membrane matrix, and mimics the breast cancer environment, it said.
The study highlighted a new way of targeting cancer cells by manoeuvring nanomotors inside a tumour and waiting for them to localise in the vicinity of the cancerous site.
"We tried driving the nanomotors toward cancer cells in a tumour model and observed them getting stuck to the matrix near cancer cells, but this was not observed near normal cells," says Debayan Dasgupta, a co-first author and PhD student at CeNSE.
The extracellular matrix (ECM) is a complex 3D network of proteins and carbohydrates secreted by living cells into their neighbourhood.
A helical nanomotor under a scanning electron microscope. Image: Wikimedia Commons
However, when cancer cells secrete fresh material into the ECM, it disrupts the chemical and physical composition of the native ECM surrounding healthy cells, degrading the local environment.
"Therefore, understanding how the cellular microenvironment is altered due to cancer cells and measuring these changes quantitatively could be vital in understanding the progression of cancer," IISc noted.
In the current study, the researchers discovered that as the nanomotors approached the cancer cell membrane, they stuck to the matrix more strongly than they would to normal cells.
To measure how strongly the nanomotors bound to the matrix, the team calculated the magnetic field strength required to overcome the adhesive force, and move forward, it was stated.
"This means that the cancer cells are doing something.
So, we did some measurements and discovered that it [the adhesive force] depended on the type of cells, the strength of interaction and also which side of the cell the nanomotor approached," explains Ambarish Ghosh, Associate Professor at CeNSE and one of the senior authors.
"In the end, we really ended up discovering a physical property of an important biological environment."
"The reason why the nanomotors appear to stick to the cancer cells better is their charged ECM. This may be due to the presence of 2,3-linked sialic acid, a sugar-conjugated molecule which confers a negative charge on the cancer cell environment, the researchers found.
They visualized the distribution of these sugars using fluorescent markers and found that sialic acids were distributed up to 40 micrometres from the cancer cell surface – the same distance until which the nanomotors experienced strong adhesion, the statement said.
To counter this adhesive effect, the team coated the nanomotors with Perfluorooctyltriethoxysilane (PFO) which shielded them from the charged environment.
The coated nanomotors did not stick to the matrix near the cancer cells, whereas the uncoated motors clung to the matrix, confirming the fact that the negatively charged cancer microenvironment interacts with the incoming nanomotors, rendering them immobile.
"What came as a beautiful surprise was that within such a milieu, we found that aggressive cancer cells ended up remodelling their surroundings by making them stickier, and richer in specific charged sugars," says Ramray Bhat, Assistant Professor at MRDG and one of the senior authors.
"This charging can potentially be used to target and kill tiny populations of cancer cells hidden among their normal counterparts, for which we are extending these studies to living animals", Bhat added.
Catalase, a commonly used low-cost enzyme, holds potential as a therapeutic drug to treat COVID-19 symptoms, and suppress the reproduction of the novel coronavirus inside the body, according to a study.
Catalase is produced naturally and used by humans, animals, and plants. Inside cells, the enzyme kick starts the breakdown of hydrogen peroxide, which can be toxic, into water and oxygen.
The antioxidant enzyme is also commonly used worldwide in food production and as a dietary supplement.
"There is a lot of focus on vaccines and antiviral drugs, and rightly so," said Yunfeng Lu from the University of California, Los Angeles (UCLA) in the US.
"In the meantime, our research suggests this enzyme could offer a very effective therapeutic solution for treatment of hyperinflammation that occurs due to SARS-CoV-2 virus, as well as hyperinflammation generally, said Lu, a senior author of the study published in the journal Advanced Materials.
The team, including researchers from the Chinese Academy of Medical Sciences, and Jinan University, China developed the drug-delivery technology used in the experiments.
Three types of tests were conducted, each addressing a different symptom of COVID-19.
The researchers demonstrated the enzyme's anti-inflammatory effects and its ability to regulate the production of cytokines, a protein that is produced in white blood cells.
Cytokines are an important part of the human immune system, but they can also signal the immune system to attack the body's own cells if too many are made – a so-called "cytokine storm" that is reported in some patients diagnosed with COVID-19.
The researchers also showed that catalase can protect alveolar cells, which line the human lungs, from damage due to oxidation.
The experiments showed that catalase can repress the replication of SARS-CoV-2 virus in rhesus macaques, a type of monkey, without noticeable toxicity.
"This work has far-reaching implications beyond the treatment of COVID-19. Cytokine storm is a lethal condition that can complicate other infections, such as influenza, as well as non-infectious conditions, like autoimmune disease," said Gregory Fishbein a pathologist at UCLA.
Redmi 9i with a 5,000 mAh battery debuted in India recently. This is the fourth model of the company's Redmi 9 series that already includes Redmi 9 Prime, Redmi 9 and Redmi 9A. The highlight of Redmi 9i is 4 GB RAM.
The smartphone will be available for purchase today at 12 pm in India.
The 4 GB RAM + 64 GB storage variant is priced at Rs 8,299 and the 4 GB RAM + 128 GB storage variant is priced at Rs 9,299.
It will be available in Sea Blue, Nature Green and Midnight Black colour variants.
The smartphone will go on sale today at 12 pm on Flipkart and Mi.com.
The smartphone features a 6.53-inch HD+ IPS display that has a 20:9 aspect ratio. It is powered by a MediaTek Helio G25 chipset and offers 4 GB RAM and up to 128 GB internal storage that is expandable up to 512 GB via a micro SD card. Redmi 9i runs on MIUI 12.
In terms of camera, it comes with a 13 MP rear camera setup and a 5 MP selfie camera. The camera also comes with Pro mode, Palm Shutter mode and more. According to Redmi, it also features AI scene detection which recognises 32 different scenes, including 5 India specific scenes.
It is equipped with a 5,000 mAh battery. It also comes with a P2i coating that makes it water-resistant.
Realme launched Realme 7 (Review) and Realme 7 Pro (Review) in India at a starting price of Rs 14,999 and Rs 19,999 respectively. The headlining feature of the Realme 7 series is the 64 MP quad camera setup.
Realme 7 will be available for purchase today at 12 pm on Flipkart.
Realme 7 comes in two storage variants. The 6 GB RAM + 64 GB storage variant is priced at Rs 14,999 and the 8 GB RAM + 128 GB storage variant is priced at Rs 16,999.
It comes in Mist Blue and Mist White colour variants.
You can buy the phone today at 12 pm on Flipkart and Realme.com.
Realme 7 features a 6.5-inch full-HD+ display. It comes with a side-mounted fingerprint scanner. It is powered by MediaTek Helio G95 processor and offers up to 8 GB RAM and 128 GB storage variants.
In terms of camera, it comes with a quad rear camera setup that includes 64 MP Sony IMX682 primary sensor, 8 MP ultra-wide-angle lens, 2 MP macro lens and a 2 MP lens.
It sports a 16 MP punch hole selfie camera.
Realme 7 is equipped with a 5,000 mAh battery that supports 30W Dart fast charging tech.
 Former India captain Mahendra Singh Dhoni and his wife Sakshi plan to score in a very different game than cricket. They are foraying into OTT space with a web series. Sakshi and MSD launched their banner Dhoni Entertainment in 2019 with the documentary "Roar Of The Lion". Now, they are coming up with a web series, which is an adaptation of an unpublished book written by a debutant author. Sakshi spoke to IANS on the Dhonis' decision to venture into the entertainment world,
Former India captain Mahendra Singh Dhoni and his wife Sakshi plan to score in a very different game than cricket. They are foraying into OTT space with a web series. Sakshi and MSD launched their banner Dhoni Entertainment in 2019 with the documentary "Roar Of The Lion". Now, they are coming up with a web series, which is an adaptation of an unpublished book written by a debutant author. Sakshi spoke to IANS on the Dhonis' decision to venture into the entertainment world, Actress Trisha Krishnan's life changed when 21 years ago she was crowned Miss Chennai. September 30th will always remain special for the actress as it was the same day when won the crown. On Wednesday, Trisha went down the memory lane and shared a picture of one of her most important days in life. "30.9.1999 - The day my life changed. Miss Chennai 1999," she wrote. Fans and friends flooded the actress's comments with beautiful compliments. Actress Lakshmi Machu wrote, "The only thing different I see of you now is your lip colour... you haven't changed one bit." Actress Varalaxmi Sarathkumar commented: "I remember this day."
Actress Trisha Krishnan's life changed when 21 years ago she was crowned Miss Chennai. September 30th will always remain special for the actress as it was the same day when won the crown. On Wednesday, Trisha went down the memory lane and shared a picture of one of her most important days in life. "30.9.1999 - The day my life changed. Miss Chennai 1999," she wrote. Fans and friends flooded the actress's comments with beautiful compliments. Actress Lakshmi Machu wrote, "The only thing different I see of you now is your lip colour... you haven't changed one bit." Actress Varalaxmi Sarathkumar commented: "I remember this day." Brie Larson has started her YouTube channel which attracts criticism from trolls every now and then. However, Brie is not one to take hatred lying down.
Brie Larson has started her YouTube channel which attracts criticism from trolls every now and then. However, Brie is not one to take hatred lying down. Bollywood actress Kangana Ranaut is all set to resume work after a long break of seven months. The actress was staying with her family in Manali during the COVID19 pandemic. Now that the lockdown has been lifted that everyone is back at work keeping in mind all the safety precautions, Kangana also gears up to travel to South India to complete her most awaited film Thalaivi. The film is a biopic of Former Chief Minister of Tamil Nadu, J. Jayalalithaa. Kangana had already shared her first look from the film and has also been undertaking dance classes to ace the leader's persona to perfection.
Bollywood actress Kangana Ranaut is all set to resume work after a long break of seven months. The actress was staying with her family in Manali during the COVID19 pandemic. Now that the lockdown has been lifted that everyone is back at work keeping in mind all the safety precautions, Kangana also gears up to travel to South India to complete her most awaited film Thalaivi. The film is a biopic of Former Chief Minister of Tamil Nadu, J. Jayalalithaa. Kangana had already shared her first look from the film and has also been undertaking dance classes to ace the leader's persona to perfection. A recent survey has revealed that knitting can reduce stress levels and heart rate, claiming it to be the best hobby one can take up during COVID-19 pandemic.
A recent survey has revealed that knitting can reduce stress levels and heart rate, claiming it to be the best hobby one can take up during COVID-19 pandemic. Pandemic had influence and change the course of history multiple times. Many have killed a large percentage of the human population over the ages.
Pandemic had influence and change the course of history multiple times. Many have killed a large percentage of the human population over the ages. Reusing and recycling the plastic waste into something useful and fashionable will helpbreduce the plastic piling up in the world. Take a look at some of these DIY projects.
Reusing and recycling the plastic waste into something useful and fashionable will helpbreduce the plastic piling up in the world. Take a look at some of these DIY projects. The data was collected between April 25 and May 20 from 15 hospitals in Maharashtra. It represented the pregnant women in labour or who were expected to deliver in the next five days.
The data was collected between April 25 and May 20 from 15 hospitals in Maharashtra. It represented the pregnant women in labour or who were expected to deliver in the next five days. PCOS can cause many symptoms including an irregular menstrual cycle, acne, hair problems and weight gain.
PCOS can cause many symptoms including an irregular menstrual cycle, acne, hair problems and weight gain. Take a look at some of the lesser-known yet amazing dance forms that explore the rich culture of the country.
Take a look at some of the lesser-known yet amazing dance forms that explore the rich culture of the country. A viral throwback picture of Priyanka Chopra shows her cheering for Nick Jonas who played football with the All Stars Football Club at Bandra, Mumbai back in 2018.
A viral throwback picture of Priyanka Chopra shows her cheering for Nick Jonas who played football with the All Stars Football Club at Bandra, Mumbai back in 2018. Bollywood actor Sushant Singh Rajput's fans and family have been awaiting justice in his death case. While the Central Bureau of Investigation (CBI) is investigating the final report submitted by the All India Institute of Medical Sciences (AIIMS) Forensic Department, the Narcotics Control Bureau (NCB) is looking into the seized mobile phones of over 45 people including Bollywood celebrities Deepika Padukone, Sara Ali Khan, Shraddha Kapoor, Rhea Chakraborty, DP's manager Karishma and others. It is said that the investigation will lead to more big names coming to the fore and NCB will summon three male stars of the film industry soon for questioning. Earlier, it was rumoured that NCB has 'almost' given clean chit to the Bollywood divas Deepika, Shraddha, Sara but the agency refuted the claims saying such reports are "devoid" of "truth and facts".
Bollywood actor Sushant Singh Rajput's fans and family have been awaiting justice in his death case. While the Central Bureau of Investigation (CBI) is investigating the final report submitted by the All India Institute of Medical Sciences (AIIMS) Forensic Department, the Narcotics Control Bureau (NCB) is looking into the seized mobile phones of over 45 people including Bollywood celebrities Deepika Padukone, Sara Ali Khan, Shraddha Kapoor, Rhea Chakraborty, DP's manager Karishma and others. It is said that the investigation will lead to more big names coming to the fore and NCB will summon three male stars of the film industry soon for questioning. Earlier, it was rumoured that NCB has 'almost' given clean chit to the Bollywood divas Deepika, Shraddha, Sara but the agency refuted the claims saying such reports are "devoid" of "truth and facts".  TV personality Teejay Sidhu, who is currently expecting her third child, has opened up on how she is being called too skinny while being pregnant. Taking to Instagram, Teejay posted a picture flaunting her baby bump, and requested all expecting mothers to love their maternity body.
TV personality Teejay Sidhu, who is currently expecting her third child, has opened up on how she is being called too skinny while being pregnant. Taking to Instagram, Teejay posted a picture flaunting her baby bump, and requested all expecting mothers to love their maternity body.We are the midst of the Honda two-wheeler launch in India. And the motorcycle will be called the CB 350. From the looks of it, the Honda CB 350 will rival the likes of the Royal Enfield Classic 350 and also the Benelli Imperiale 400 in India. This is the first cruiser motorcycle by Honda in India. It will be manufactured in India and will be sold through the company's Honda Big Wing India network, giving it a premium appeal. The Honda CB 350 has been priced at Rs 1.9 lakh, ex-showroom.
Honda CB 350 cruiser.
From the images, it can be seen that the Honda CB 350 comes with a retro design that the CB brand has been famous for. The details include neo-classic LED headlights, single-pod instrumentation, alloy wheels and interesting chrome highlights all through the design of the motorcycle. The motorcycle will come with a dual-channel ABS as standard. Further, the motorcycle gets chrome mirrors, voluptuous tank design and a single seat that looks reasonably comfortable for longer saddle time.
The Honda CB 350 will be powered by a single-cylinder 350cc motor which should make an impressive power and torque rating. Details of which will be released shortly.
 A prequel to "The Lion King" is in development at Disney Studios with Oscar winner Barry Jenkins attached to direct the new project. According to Deadline, Jeff Nathanson, who worked on the Jon Favreau-directed film last year, has penned a draft of the prequel. "The Lion King", a remake of the 1994 animated classic, used innovative techniques to create photorealistic animals and African landscapes. Voice starring Donald Glover as Simba and Beyonce as Nala, the film became a massive hit for the studio.
A prequel to "The Lion King" is in development at Disney Studios with Oscar winner Barry Jenkins attached to direct the new project. According to Deadline, Jeff Nathanson, who worked on the Jon Favreau-directed film last year, has penned a draft of the prequel. "The Lion King", a remake of the 1994 animated classic, used innovative techniques to create photorealistic animals and African landscapes. Voice starring Donald Glover as Simba and Beyonce as Nala, the film became a massive hit for the studio. It seems that Bigg Boss 14 is going to be a 'dhamakedaar' season as the makers have planned the entry of super controversial contestants this time. Just yesterday a new promo of self-styled Godwoman Radhe Maa was shared that left the fans intrigued about what will the courses of events in the show. Her entry in Salman Khan's reality show is now confirmed as she can be seen taking a walkthrough in the BB house with her very popular 'flute' in her hand. As devotional music plays in the background, Radhe Maa can be heard saying, "Ye ghar humesha bana rahe, Bigg Boss is bar bohot chale." Have a look at some of her viral photos and videos that caught everyone's attention.
It seems that Bigg Boss 14 is going to be a 'dhamakedaar' season as the makers have planned the entry of super controversial contestants this time. Just yesterday a new promo of self-styled Godwoman Radhe Maa was shared that left the fans intrigued about what will the courses of events in the show. Her entry in Salman Khan's reality show is now confirmed as she can be seen taking a walkthrough in the BB house with her very popular 'flute' in her hand. As devotional music plays in the background, Radhe Maa can be heard saying, "Ye ghar humesha bana rahe, Bigg Boss is bar bohot chale." Have a look at some of her viral photos and videos that caught everyone's attention.OnePlus CEO Pete Lau has confirmed that OnePlus 8T Pro will not debut in 2020. The announcement was made via a Weibo post where he also revealed the date for the China release of the OnePlus 8T. According to Lau, people looking for "Pro" or a flagship OnePlus phone should opt for OnePlus 8 Pro (Review) as for now. In addition to this, he revealed that there is more to look forward to than the OnePlus 8T on 14 October. According to a report by GSMArena, OnePlus might launch a smartwatch, wireless earbuds and a Nord handset in the US on 14 October.
OnePlus has also announced that OnePlus 8T will launch in India on 14 October. The smartphone will feature a 120Hz Fluid AMOLED display and will come with support for 65W Warp charging tech.
According to a previous report, the smartphone might feature a 6.55-inch display that offers a 120 Hz refresh rate. As per the renders shared in the report, OnePlus 8T is likely to sport a punch-hole display. It is expected to be powered by a Snapdragon 865 chipset and offer up to 12 GB RAM and up to 256 GB of internal storage.
As for the camera, the report reveals that the rear quad camera setup placed in a rectangular module is expected to house a 48 MP primary sensor, a 16 MP ultra-wide lens, a 5 MP macro lens and a 2 MP portrait sensor. For selfies, OnePlus 8T might come with a 32 MP front camera.
OnePlus 8T is expected to be equipped with a 4,500 mAh battery that supports 65W Warp Charge.
The Indian Council of Medical Research (ICMR) has found in its second nationwide sero survey for antibodies against SARS-CoV-2, that over seven percent of India's adult population seems to have been exposed to the coronavirus before the end of August. The survey was done between 17 August and 22 September, and its results were shared by the Union Health Ministry on Tuesday. This is a fair jump from the 0.73 percent exposure recorded in the first survey – conducted in the same 700 villages and urban wards as the second serosurvey.
Blood samples from 29,082 people were tested to look for IgG antibodies, which indicates exposure to the novel coronavirus at the population level. The sero survey is part of a larger effort to understand how widespread the prevalence of COVID-19 is in the Indian population.
–A considerable section of the Indian population has not yet been exposed to the virus and remains at varying degree of COVID-19 infection risk, depending on where they live
–One in 15 people (10 years of age or older) is likely to have been exposed to the coronavirus (SARS-CoV-2) by end of August.
–The risk of infection was twice as high in slums (15.6 percent seroprevalence) as non-slum areas (8.2 percent seroprevalence) in urban centres, and four times higher than in rural areas (4.4 percent seroprevalence).
–The seropositivity in various strata of society were dramatically different from what the first survey reported. The first survey found a much higher seropositivity in rural areas (69.4 percent) compared to urban slums (15.9 percent) or non-slums (14.6 percent). That said, the findings from the first survey are somewhat compromised, since only a quarter (25.9 percent) of the clusters surveyed were located in urban areas.
–There was lower infection-to-case ratio in August compare to May – a reflection of a substantial increase in testing and detection across the country, the ministry said.
–For every confirmed COVID-19 case in August there were 26 to 32 infections that went undetected. In May, the same figure was 81-130 undetected for every case diagnosed – a success that the government claims is due to better contact tracing and tracking for COVID-19.
Mumbai
Mumbai showed the highest seroprevalence of any city in India, with 57.8 percent of samples (from slums) positive for SARS-CoV-2 antibodies. This figure dropped to 17.4 percent for samples from non-slum areas.
Delhi
Delhi recorded a 29.1 percent seroprevalence in the second round of sampling that was conducted between 1-7 August. This was higher than the 23.1 percent recorded in the first round conducted between 27 June and 10 July.
Puducherry
Puducherry was next with a seroprevalence of 22.7 percent in the second sero survey (10-16 September). The Union Territory has recorded some 27,000 confirmed cases, 515 are deaths and 5,014 active cases till date. Puducherry's seroprevalence has seen a rapid rise from only 4.9 percent in the first round of survey (11-16 August).
Chennai, Ahmedabad, Indore
Chennai recorded a seroprevalence of 21.5 percent and Ahmedabad 17.6 percent, with Indore in Madhya Pradesh registering 7.8 percent. Each of these figures correspond to two different sero surveys that were conducted independently of each other.
The ministry warned that with the holiday season around the corner and the many festivals that will be celebrated, mass gatherings need to be strictly avoided. State governments need to come up with "inventive containment strategies" for this, said the central government.
The sero survey also highlighted the need for the public to practise 'non-pharmacological interventions' – social distancing, correct cough etiquette, use of face masks and hand sanitizers – to limit the spread of COVID-19.
Elderly people, individuals with co-morbidities, children and pregnant women remain susceptible to infection and still need to take extra precautions wherever possible, the government stressed.
India has a cumulative tally of 61.45 lakh confirmed COVID-19 cases and 9.4 lakh active cases as of 30 September. 70,589 new cases were reported in the past 24 hours, as per government data released this morning, and 776 people died.
 After a Bollywood actress came forward and accused filmmaker Anurag Kashyap of sexual harassment, many fingers were pointed at him but no action was taken yet. The actress has filed a case against him on rape charges last week and demanded justice. Now, the Mumbai Police has summoned film director Anurag Kashyap asking him to appear at Versova Police station tomorrow on October 1, at 11 am. The development came after the actress threatened to sit on hunger strike and protest if the action has not been taken. DCP Abhishek Trimukhe returned to work on Wednesday after recovering from COVID19 and held a meeting to decide when to call the filmmaker for questioning.
After a Bollywood actress came forward and accused filmmaker Anurag Kashyap of sexual harassment, many fingers were pointed at him but no action was taken yet. The actress has filed a case against him on rape charges last week and demanded justice. Now, the Mumbai Police has summoned film director Anurag Kashyap asking him to appear at Versova Police station tomorrow on October 1, at 11 am. The development came after the actress threatened to sit on hunger strike and protest if the action has not been taken. DCP Abhishek Trimukhe returned to work on Wednesday after recovering from COVID19 and held a meeting to decide when to call the filmmaker for questioning. Actress Priyanka Chopra has come a long way in her career and now she is all set to launch with her memoir named Unfinished. She recently took to her Instagram handle to tease her fans with two short video clips which give a glimpse of her journey from childhood till winning the Miss World title. She wrote the caption of the video saying, “#unfinished.” The short clip features a childhood pic of the actress where she is seen posing for the camera and another pic is of her Miss World pageant crowning where she is giving a flying kiss.
Actress Priyanka Chopra has come a long way in her career and now she is all set to launch with her memoir named Unfinished. She recently took to her Instagram handle to tease her fans with two short video clips which give a glimpse of her journey from childhood till winning the Miss World title. She wrote the caption of the video saying, “#unfinished.” The short clip features a childhood pic of the actress where she is seen posing for the camera and another pic is of her Miss World pageant crowning where she is giving a flying kiss.  Megastar Amitabh Bachchan on Wednesday revealed that he has pledged to donate his organs. The 77-year-old actor took to Instagram to share a picture of himself from the sets of his popular quiz show 'Kaun Banega Crorepati,' where he is seen sporting a green coloured ribbon on his suit. The veteran actor who is quite active on social media when on to explain the significance of the "green ribbon" in the caption and said that he is a "pledged organ donor."
Megastar Amitabh Bachchan on Wednesday revealed that he has pledged to donate his organs. The 77-year-old actor took to Instagram to share a picture of himself from the sets of his popular quiz show 'Kaun Banega Crorepati,' where he is seen sporting a green coloured ribbon on his suit. The veteran actor who is quite active on social media when on to explain the significance of the "green ribbon" in the caption and said that he is a "pledged organ donor."Motorola launched the Moto E7 Plus in India last week at a price of Rs 9,499. This smartphone series already includes Moto E6s (Review) that was launched in India last year at a starting price of Rs 7,999. The headlining features of Moto E7 Plus include Qualcomm Snapdragon 460 chipset, a 48 MP dual camera setup, and a 5,000 mAh battery with supports for 10W charging. Moto E7 Plus competes against Xiaomi's Redmi 9 Prime and Realme Naro 20A and more. Moto E7 Plus was first unveiled in Brazil earlier this month.
The smartphone comes in just one storage variant that offers 4 GB RAM and 64 GB internal storage that is expandable up to 512 GB. It is priced at Rs 9,499.
In terms of colours, it comes in Misty Blue and Twilight Orange colour variants.
The smartphone will be available for purchase today at 12 pm on Flipkart.
Moto E7 Plus features a 6.5-inch HD+ display that houses a waterdrop notch at the top. It is powered by the Snapdragon 460 chipset and offers 4 GB RAM and 64 GB internal storage that is expandable up to 512 GB. It runs on Android 10 and sports a rear-mounted fingerprint scanner. The smartphone also comes with a dedicated Google Assistant button.
In terms of camera, the smartphone comes with a dual rear camera setup packed in a square camera module. This setup houses a 48 MP primary sensor and a 2 MP depth sensor. On the front, it comes with an 8 MP selfie camera.
Moto E7 Plus is equipped with a 5,000 mAh battery that supports 10W charging.
It has been almost a month since the government banned the PUBG Mobile game in India. Many attempts by authorities are being made so that the much popular game makes it's way back to India but nothing has worked quite in their favour till now. These attempts include PUBG Corporation cutting ties with Tencent for PUBG Mobile in India. A recent report by InsideSport revealed that the Ministry of Electronics and Information Technology has confirmed that the PUBG ban is now permanent in India.
According to a report by Reuters, despite PUBG Corporation taking over the publishing rights in India, the government is not yet ready to revoke the ban. A source informed Reuters that the government is not ready to lift the ban not because it is a Chinese app but because the game is violent.
As per the report, the source said, "The violent nature of the game has been the cause of many complaints from all quarters. That does not change with the change in ownership rights.”
The report further reveals that PUBG Corporation is now looking into India's concerns and "is ready to work on anything that needed improvement". The company is reportedly in touch with Jio Platforms "to seek for cooperation opportunities" but nothing has been finalised yet.
Realme launched the Realme C11 at a price of Rs 7,499. Today, the smartphone will be available for purchase at 2 pm on Flipkart and the company's website. The highlight of Realme C11 includes its MediaTek Helio G35 chipset and a 5,000 mAh battery.
Realme C11 comes in just one storage variant that offers 2 GB RAM and 32 GB of internal storage and is priced at Rs 7,499. It comes in Rich Green and Rich Grey colour variants.
The smartphone will be available for purchase today at 2 pm on Flipkart and Realme's website.
The smartphone features a 6.5-inch IPS-matrix display with a resolution of HD+ (1600×720 pixels). It sports a 5 MP front camera with an f/2.4 aperture. Besides, Realme C11 has a 13 MP (f/2.2) dual main camera + 2 MP (portrait, f/2.4) with Super Nightscape night mode.
Powered by a Helio G35 chip, the phone is equipped with a 5,000 mAh battery. It features a Bluetooth 5.0 module and a 3.5 mm headphone jack. Running Android 10 OS with a Realmi UI interface, it comes with no fingerprint scanner.
Equipped with face unlock feature, Realme C11 weighs 196 grams.
 Sutapa Sikdar, wife of late actor Irrfan Khan, has made an appeal for the legalisation CBD oil in India. Sutapa took to her unverified Instagram account on Tuesday to present her viewpoint. Sharing a photograph of the London hospital where Irrfan Khan underwent cancer treatment, Sutapa wrote on Instagram: "London revisit looking at his hospital room from outside like everytime I did while he was there #walkingalone #wishyouwerethere #cancerpain #LegalizeCBDoilinindia."
Sutapa Sikdar, wife of late actor Irrfan Khan, has made an appeal for the legalisation CBD oil in India. Sutapa took to her unverified Instagram account on Tuesday to present her viewpoint. Sharing a photograph of the London hospital where Irrfan Khan underwent cancer treatment, Sutapa wrote on Instagram: "London revisit looking at his hospital room from outside like everytime I did while he was there #walkingalone #wishyouwerethere #cancerpain #LegalizeCBDoilinindia." Rap superstar Yo Yo Honey Singh has been around for a decade and a half now, and he says there has been a lot of change in Punjabi and Hindi rap during this tenure. "Evolution happens in everything, be it music, lifestyle or writing -- anything. Evolution comes with time and it would be good if you adapt yourself with the evolution," Honey Singh told IANS.
Rap superstar Yo Yo Honey Singh has been around for a decade and a half now, and he says there has been a lot of change in Punjabi and Hindi rap during this tenure. "Evolution happens in everything, be it music, lifestyle or writing -- anything. Evolution comes with time and it would be good if you adapt yourself with the evolution," Honey Singh told IANS. Not many days are left when Bigg Boss season 14 is about to begin its journey. And as the date of the show is nearing the makers are hardly leaving any stone unturned to make the audience more curious. A lot of celebrities are rumoured to be entering the house. But very few of them have confirmed their presence in this high voltage reality show and one such name is that of Nikki Tamboli. Recently, the show has released the teaser of Bigg Boss 14 featuring someone who looks like the South actress. The video shows Nikki shaking a leg on Nora Fetehi’s Dilbar song.
Not many days are left when Bigg Boss season 14 is about to begin its journey. And as the date of the show is nearing the makers are hardly leaving any stone unturned to make the audience more curious. A lot of celebrities are rumoured to be entering the house. But very few of them have confirmed their presence in this high voltage reality show and one such name is that of Nikki Tamboli. Recently, the show has released the teaser of Bigg Boss 14 featuring someone who looks like the South actress. The video shows Nikki shaking a leg on Nora Fetehi’s Dilbar song. When they were asked who is the best cook in the house, Akshay Kumar quipped that he is the best as wife Twinkle Khanna cannot even make an omelette.
When they were asked who is the best cook in the house, Akshay Kumar quipped that he is the best as wife Twinkle Khanna cannot even make an omelette.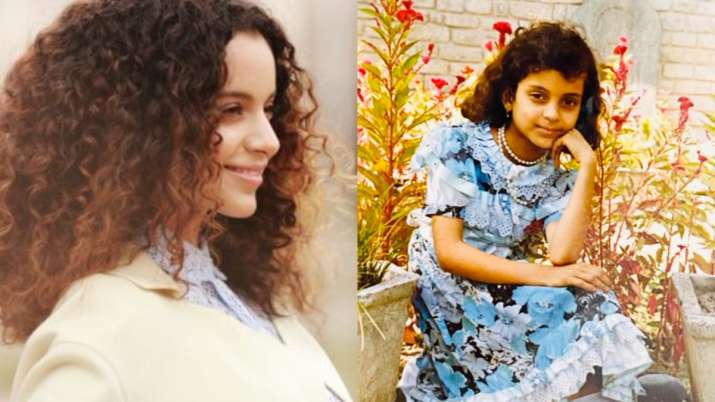 Bollywood actress Kangana Ranaut has carved a niche for herself in the film industry. Not just for her stellar performances, the actress is known as a fashion icon and steals the show in every public appearance. On Wednesday, Kangana went down the memory lane and shared pictures from her childhood when she used to dress up. The actress revealed that she used to wear ornaments and cut her own hair and was often laughed at in her childhood. However, later she earned a place in the front rows of the top fashion shows.
Bollywood actress Kangana Ranaut has carved a niche for herself in the film industry. Not just for her stellar performances, the actress is known as a fashion icon and steals the show in every public appearance. On Wednesday, Kangana went down the memory lane and shared pictures from her childhood when she used to dress up. The actress revealed that she used to wear ornaments and cut her own hair and was often laughed at in her childhood. However, later she earned a place in the front rows of the top fashion shows.Realme launched the Narzo 20 series that includes Narzo 20A, Narzo 20 and Narzo 20 Pro (Review) recently in India. Today, the least expensive smartphone of the series, Narzo 20A, will go on its first sale on Flipkart and Realme.com. The highlights of this smartphone include its triple rear camera setup, 5,000 mAh battery and 4 GB RAM. The smartphone comes in two storage variants that offer 32 GB and 64 GB internal storage.
The Realme Narzo 20A 3 GB RAM + 32 GB storage variant is priced at Rs 8,499 and the 4 GB RAM + 64 GB is priced at Rs 9,499. The smartphone comes in Victory Blue and Glory Silver colour variants.
The Victory Design of #realmeNarzo20A is as attractive in pictures as in real life.
Glory Silver or Victory Blue?
What’s your pick?Sale tomorrow at 12 PM only on https://t.co/HrgDJTZcxv & @Flipkart.
Know more: https://t.co/OSmN1jU8JV pic.twitter.com/wnOLJ00NMt— realme (@realmemobiles) September 29, 2020
It will go on sale for the first time today at 12 pm on Flipkart and Realme's website.
Realme Narzo 20A features a 6.5-inch HD+ display. It is powered by Snapdragon 665 chipset and offers up to 4 GB RAM and up to 64 GB of internal storage.
The smartphone comes with a triple rear camera setup that includes 12 MP primary sensor, a 2 MP monochrome sensor and a 2 MP "retro" sensor. For selfies, it features an 8 MP front camera.
The smartphone comes with a 5,000 mAh battery that supports reverse charging.
 Popular comedy show Taarak Mehta Ka Ooltah Chashmah fame TV actress Priya Ahuja tested positive for COVID19. The actress took to her social media to inform about it to her fans and revealed that she has been following the rules and is in home quarantine. The actress wrote, "It’s my duty to inform you all that I have been tested COVID POSITIVE...I’m asymptotic I’m doing okay!...I’m following instructions provided by doctors n BMC I’m in home quarantine...Incase if any of you came in touch with me in last 2-3 days get yourself tested pls...I haven’t been shooting n was at home all this while still got this virus.. keep ur self safe n don’t forget to wear the mask..Don’t take it lightly."
Popular comedy show Taarak Mehta Ka Ooltah Chashmah fame TV actress Priya Ahuja tested positive for COVID19. The actress took to her social media to inform about it to her fans and revealed that she has been following the rules and is in home quarantine. The actress wrote, "It’s my duty to inform you all that I have been tested COVID POSITIVE...I’m asymptotic I’m doing okay!...I’m following instructions provided by doctors n BMC I’m in home quarantine...Incase if any of you came in touch with me in last 2-3 days get yourself tested pls...I haven’t been shooting n was at home all this while still got this virus.. keep ur self safe n don’t forget to wear the mask..Don’t take it lightly." Actress Divya Dutta, who has completed 26 years in the entertainment industry, celebrated her birthday quietly with loved ones at home on September 25. Divya started out as a mainstream Bollywood actress in the mid-nineties but made heads turn for the first time with a small but impactful role in Pamela Rooks' 1998 screen adaptation of Khushwant Singh's novel, "Train To Pakistan". Over the years, she has carved a niche as a vesatile performer who can impress in commercial as well as arthouse cinema.
Actress Divya Dutta, who has completed 26 years in the entertainment industry, celebrated her birthday quietly with loved ones at home on September 25. Divya started out as a mainstream Bollywood actress in the mid-nineties but made heads turn for the first time with a small but impactful role in Pamela Rooks' 1998 screen adaptation of Khushwant Singh's novel, "Train To Pakistan". Over the years, she has carved a niche as a vesatile performer who can impress in commercial as well as arthouse cinema. Bigg Boss 13 fame Himanshi Khurana recently took to her social media to inform her fans that she has tested COVID19 positive. The Punjabi actress and singer wrote, "I want to inform you all that I have tested positive for COVID-19 even after taking proper precautions. As you all know that I was the part of the protest day before yesterday and the area was crowded so I thought to get the test done before I go for my shoot today evening. I just wanted to inform people who came in my contact to get your test done and please take proper precaution in the protest. It's my request to all the people protesting to not forget that we are going through pandemic so please take proper care " She has joined the farmers in Punjab in their protest against the Farm Bill.
Bigg Boss 13 fame Himanshi Khurana recently took to her social media to inform her fans that she has tested COVID19 positive. The Punjabi actress and singer wrote, "I want to inform you all that I have tested positive for COVID-19 even after taking proper precautions. As you all know that I was the part of the protest day before yesterday and the area was crowded so I thought to get the test done before I go for my shoot today evening. I just wanted to inform people who came in my contact to get your test done and please take proper precaution in the protest. It's my request to all the people protesting to not forget that we are going through pandemic so please take proper care " She has joined the farmers in Punjab in their protest against the Farm Bill. Indian playback singer Shaan turns 48 on September 30, 2020. On his birthday, let us look some of his most memorable numbers that people absolutely love.
Indian playback singer Shaan turns 48 on September 30, 2020. On his birthday, let us look some of his most memorable numbers that people absolutely love. The Narcotics Control Bureau (NCB) is widening its approach in the drug prove in connection to Sushant Singh Rajput's death case as new names are coming into the fore. After Bollywood divas Deepika Padukone, Sara Ali Khan, Shraddha Kapoor and Rakul Preet Singh, three male actors' names have surfaced in the investigation who will be soon summoned by the NCB for questioning. The agency has seized the mobile phones of the actresses and is looking into it to decode the Bollywood drug syndicate. On the other hand, it has already arrested more than 20 people including actress Rhea Chakraborty and brother Showik in the drugs probe. On Tuesday, Bombay High Court reserved the order in the bail pleas of the brother-sister duo.
The Narcotics Control Bureau (NCB) is widening its approach in the drug prove in connection to Sushant Singh Rajput's death case as new names are coming into the fore. After Bollywood divas Deepika Padukone, Sara Ali Khan, Shraddha Kapoor and Rakul Preet Singh, three male actors' names have surfaced in the investigation who will be soon summoned by the NCB for questioning. The agency has seized the mobile phones of the actresses and is looking into it to decode the Bollywood drug syndicate. On the other hand, it has already arrested more than 20 people including actress Rhea Chakraborty and brother Showik in the drugs probe. On Tuesday, Bombay High Court reserved the order in the bail pleas of the brother-sister duo.  The Forensic Department of the All India Institute of Medical Sciences (AIIMS), which has been roped in by the Central Bureau of Investigation (CBI) to give medico-legal opinion in Bollywood actor Sushant Singh Rajput's death case, still needs to look into some legal aspects before reaching a "logical legal conclusion". "AIIMS and CBI are in agreement on the Sushant Singh Rajput death case, but more deliberations are needed. There is a need to look into some legal aspects for a logical legal conclusion," Sudhir Gupta, Chairman of AIIMS Forensic Medical Board, told IANS. The remarks came almost a week after the AIIMS forensic team returned from Mumbai.
The Forensic Department of the All India Institute of Medical Sciences (AIIMS), which has been roped in by the Central Bureau of Investigation (CBI) to give medico-legal opinion in Bollywood actor Sushant Singh Rajput's death case, still needs to look into some legal aspects before reaching a "logical legal conclusion". "AIIMS and CBI are in agreement on the Sushant Singh Rajput death case, but more deliberations are needed. There is a need to look into some legal aspects for a logical legal conclusion," Sudhir Gupta, Chairman of AIIMS Forensic Medical Board, told IANS. The remarks came almost a week after the AIIMS forensic team returned from Mumbai. Heart disease is one of the prime causes of death worldwide and is mostly preventable by changing your lifestyle and managing risk factors.
Heart disease is one of the prime causes of death worldwide and is mostly preventable by changing your lifestyle and managing risk factors. An unhealthy lifestyle will eventually lead to an unhealthy heart. Here are some tips by a cardiologist to achieve a healthy heart despite having a busy work schedule.
An unhealthy lifestyle will eventually lead to an unhealthy heart. Here are some tips by a cardiologist to achieve a healthy heart despite having a busy work schedule. Actor Aftab Shivdasani is all set for his digital debut with Poison 2 and the trailer for the ZEE5 web series is out. Poison 2 will land online on October 16. The trailer shows a crime being investigated and Aditya Singh Rathore (Aftab) pulling all the strings to ensure that the trail does not lead to him.
Actor Aftab Shivdasani is all set for his digital debut with Poison 2 and the trailer for the ZEE5 web series is out. Poison 2 will land online on October 16. The trailer shows a crime being investigated and Aditya Singh Rathore (Aftab) pulling all the strings to ensure that the trail does not lead to him.